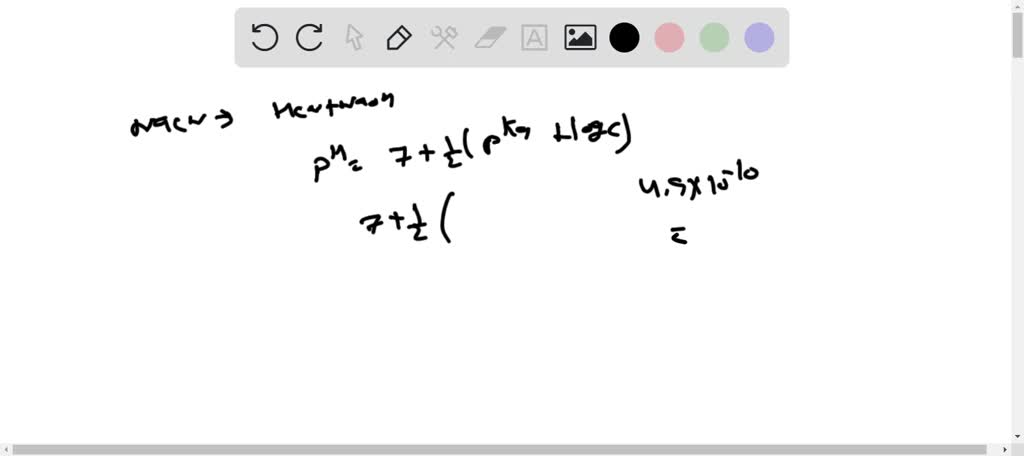
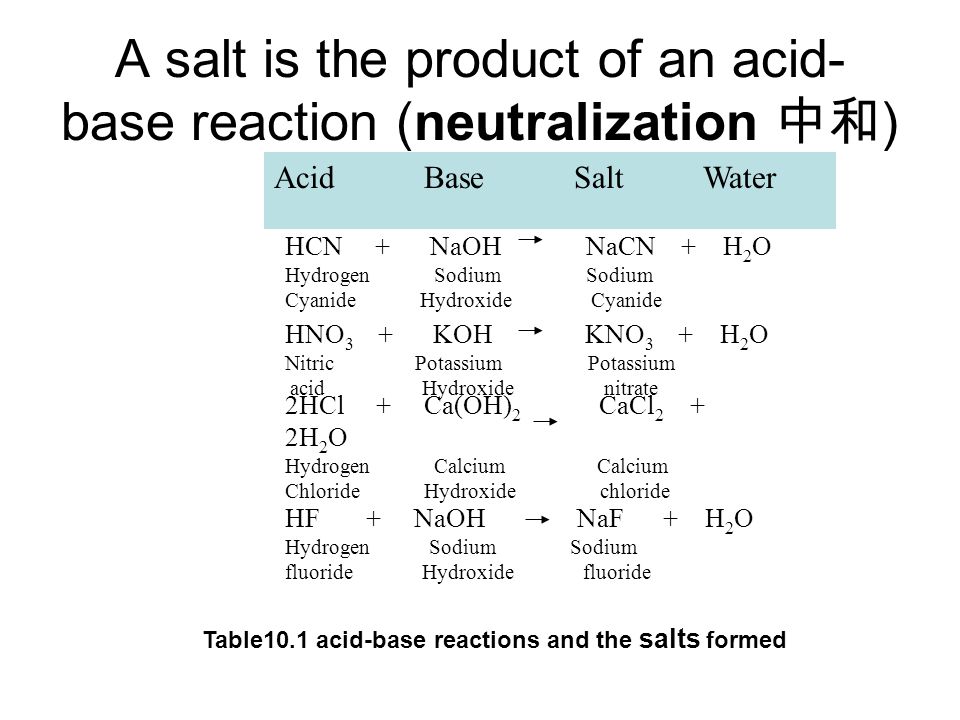
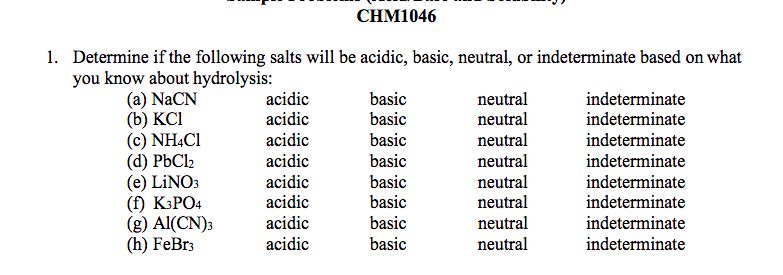
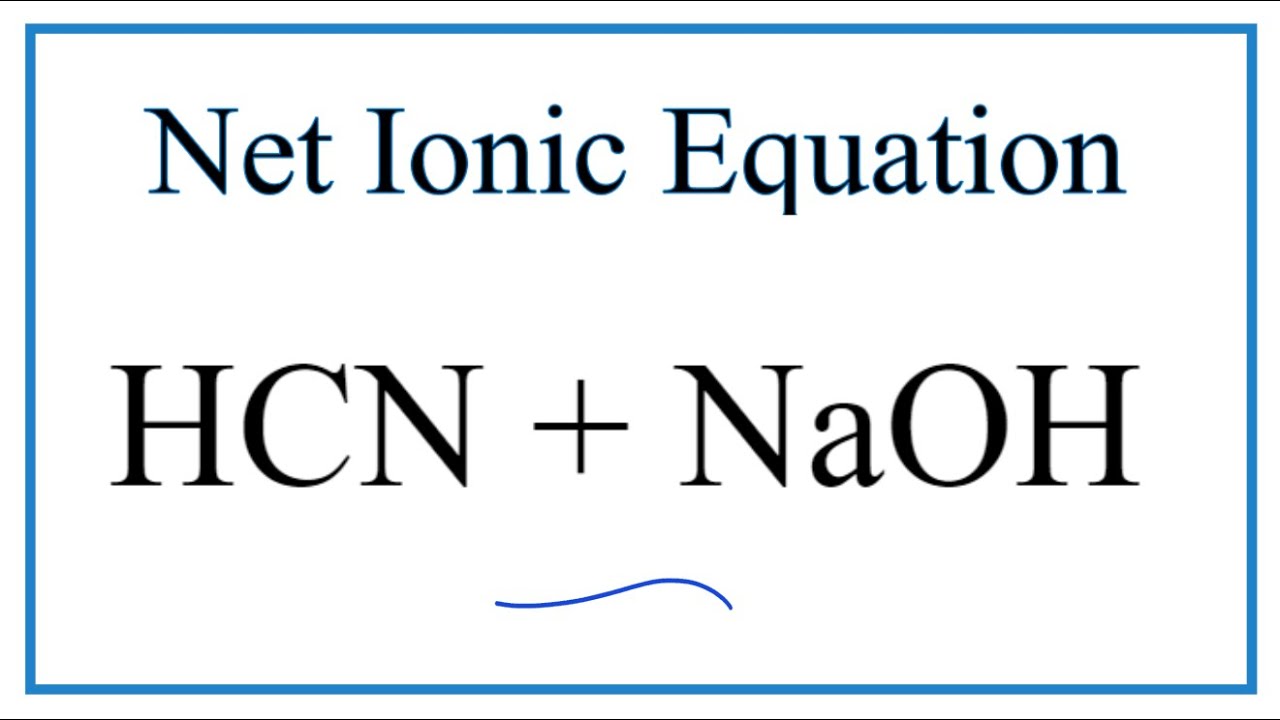Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF

Which pair of compounds will form a buffer in aqueous solution? NaCN and KCN HCl and NaOH NaCN - Home Work Help - Learn CBSE Forum
✓ Solved: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt...

Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF